 ADRe study findings into practice and improved care update
ADRe study findings into practice and improved care update
30 July 2018
Adverse drug reactions (known as ADRs) can occur both in the home, and within the healthcare setting, when combinations of medications produce unexpected side effects. Unfortunately this means that in the most serious cases fatalities can occur. However ADRe has helped all service users by addressing life-threatening problems, reducing pain or improving quality of life.
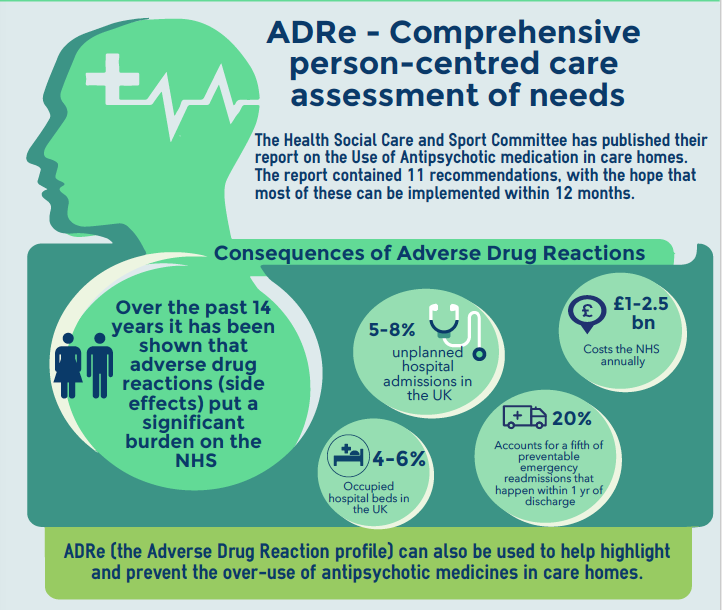
With preventable ADRs responsible for 5-8% unplanned hospital admissions in the UK, and costing the NHS up to £2.5bn pa, it is crucial that healthcare organisations take advantage of tools which can help improve how medicines are managed. ADRe has been developed with the aid of nursing professionals to help nursing staff take a structured approach to the monitoring of medicines, identifying any ADRs service users may be experiencing, and then making changes to improve a patients' health and wellbeing.
The ADRe study - Adverse Drug Reaction profile to address this. Findings from the study were included in the Welsh Government report: 'Use of Antipsychotic medication in care homes' (p.23) referred to at the Senedd on 11th July 2018 by two parties.
The report has attached short timelines (6-12 months) to most of its recommendations.
Professor Sue Jordan at Swansea University who led the study said:
"ADRe has been validated and tested, so we believe that ADRe can be quickly and easily incorporated into existing care homes procedures and monitoring systems to meet the recommendations of the Report.
We would like to express our thanks to everyone who took part in the study including the our European survey."
Further information about the study can be found at: http://www.swansea.ac.uk/adre/
Full evidence and Welsh Government response: http://senedd.assembly.wales/mgIssueHistoryHome.aspx?IId=17249 and the debate: http://record.assembly.wales/Plenary/4999#A44823 (starts p.90 & we are on pp. 93 (307), 106 (356), 112 (382).
